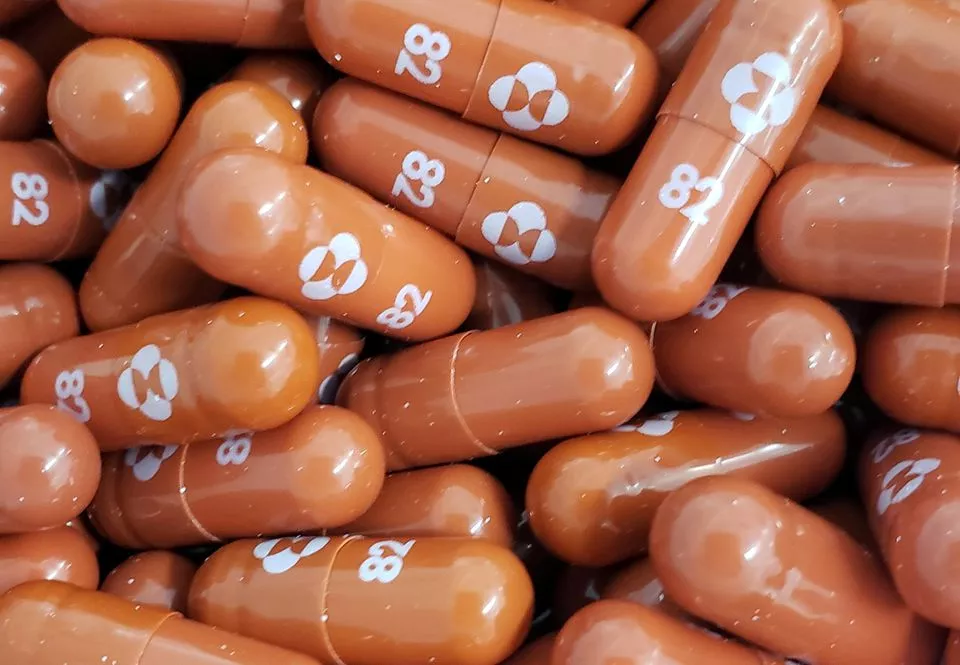
Molnupiravir, a new Covid-19 drug, reduces the risk of hospitalization and death. (Photo: Reuters)
<p>
The new antiviral pill to fight Covid-19 developed by global pharma giant Merck &amp; Co may turn out to the game-changer that the world has been looking for as it is a simple pill that can kill coronavirus.</p>
<p>
Merck and partner Ridgeback Biotherapeutics LP announced that the results were so strong that in consultation with independent trial monitors and the U.S. Food and Drug Administration, they have decided to stop enrolling more patients for the study and begin the process of gaining regulatory clearance. Merck plans to submit the data to other regulators worldwide as well.</p>
<p>
The tablet – molnupiravir – was given twice a day to patients recently diagnosed with the disease.</p>
<p>
&quot;Antiviral treatments that can be taken at home to keep people with COVID-19 out of the hospital are critically needed,&rdquo; Wendy Holman, Ridgeback&#39;s CEO, said in a statement.</p>
<p>
The drug has shown 50% efficacy in trials. In the Phase 3 trial, 7% of volunteers in the group that received the drug were hospitalized, and none died. In the group that got a placebo, 14% were hospitalized out of which some died.</p>
<p>
<strong>Also read:</strong>&nbsp;&nbsp;<a href="https://www.indianarrative.com/health-news/who-pins-hopes-on-india-resuming-vaccine-exports-as-africa-faces-crisis-114954.html">WHO pins hopes on India resuming vaccine exports as Africa faces crisis</a></p>
<p>
If it gets authorization, molnupiravir, which is designed to introduce errors into the genetic code of the virus, would be the first oral antiviral medication for COVID-19.</p>
<p>
Dr. Anthony Fauci said Friday the FDA will review data on Merck and Ridgeback Biotherapeutics&rsquo; new Covid oral antiviral quickly in hopes of issuing an emergency use authorization.</p>
<p>
<strong>India pharma firms to play key role</strong></p>
<p>
Merck had already signed non-exclusive voluntary licensing agreements with five established Indian generics manufacturers which will help to ramp up production and make the drug affordable for poor countries as well.</p>
<p>
The agreements have been signed with Cipla, Dr Reddy&rsquo;s Laboratories, Emcure Pharmaceuticals, Hetero Labs and Sun Pharmaceutical Industries which have World Health Organization (WHO) prequalified manufacturing facilities and capability as key suppliers to global and key low and middle-income countries (LMICs).</p>
<p>
This would help to win the war against Covid-19 as the vaccination rate is lagging behind in these countries.</p>
<p>
The global health agency Unitaid and its partners hope to reach an agreement as soon as next week to secure the first supplies of the antiviral treatment for lower- and middle-income nations, Philippe Duneton, its executive director, said in an interview. Unitaid has been in discussions with the company and generic manufacturers.</p>
<p>
&ldquo;This is really what we&rsquo;ve waited for all these months,&rdquo; he said. &ldquo;There is a window of hope with this treatment, and now we need to collectively make it work for people&rdquo; in less well-to-do countries, according to a Bloomberg report.</p>
Israel Defence Forces Spokesperson Brigadier General Effie Defrin on Thursday said that Iran has expressed…
India's Hindustan Aeronautics Limited (HAL) and French engine manufacturer, Safran Aircraft Engines, signed an agreement…
India has emerged as a country with the third-largest growth in power generation capacity globally…
Prime Minister Narendra Modi hailed Indian chess grandmaster Divya Deshmukh for defeating world number one…
The family of detained Baloch leader Mahrang Baloch has accused prison authorities at Quetta's Hudda…
The QS World University 2026 Rankings bring great news for our education sector, as the…